Table of Contents
- 1
- 2 CRISPR-CAS9: A REVOLUTIONARY TOOL
- 3 OTHER GENE EDITING TECHNOLOGIES
- 4 MACHINE LEARNING AND DEEP LEARNING
- 5 APPLICATIONS OF AI IN MEDICINE
- 6 THE SYNERGY OF GENE EDITING AND AI
- 7 ACCELERATING GENETIC RESEARCH
- 8 ENHANCING PRECISION MEDICINE
- 9 DRUG DEVELOPMENT
- 10 OVERCOMING BIOLOGICAL CHALLENGES
- 11 GENETIC DISEASES
- 12 CANCER
- 13 INFECTIOUS DISEASES
- 14 RARE DISEASES
- 15 REGENERATIVE MEDICINE
- 16 ETHICAL CONCERNS
- 17 SOCIAL IMPLICATIONS
- 18 TECHNICAL CHALLENGES
- 19 REGULATORY AND ETHICAL FRAMEWORKS
- 20 INTERDISCIPLINARY COLLABORATION
- 21 EDUCATION AND PUBLIC ENGAGEMENT
- 22 IMPROVING OFF-TARGET EFFECTS
- 23 ENHANCING DELIVERY SYSTEMS
- 24 HIGH-THROUGHPUT SCREENING AND AI
- 25 GERMLINE EDITING AND ITS IMPLICATIONS
- 26 ENSURING EQUITY AND ACCESS
- 27 DATA PRIVACY AND SECURITY
- 28 INTEGRATION OF MULTI-OMICS DATA
- 29 AI-DRIVEN DRUG REPURPOSING
- 30 PERSONALIZED GENE THERAPY
- 31 SICKLE CELL DISEASE AND BETA-THALASSEMIA
- 32 CANCER IMMUNOTHERAPY
- 33 RARE GENETIC DISORDERS
- 34 EMERGING APPLICATIONS AND FUTURE PROSPECTS
- 35 GENE EDITING IN VIRAL INFECTIONS
- 36 GENE EDITING AND EPIGENETIC REGULATION
- 37 AI IN MANAGING COMPLEX DISEASES AND MULTIMORBIDITY
- 38 AI-DRIVEN CLINICAL TRIALS AND GENE EDITING THERAPIES
- 39 ARTIFICIAL INTELLIGENCE IN HEALTHCARE ROBOTICS AND SURGERY
- 40 BIOENGINEERING AND ORGAN REGENERATION
- 41 GLOBAL PUBLIC HEALTH
- 42 ETHICAL OVERSIGHT AND GOVERNANCE
- 43 CONCLUSION
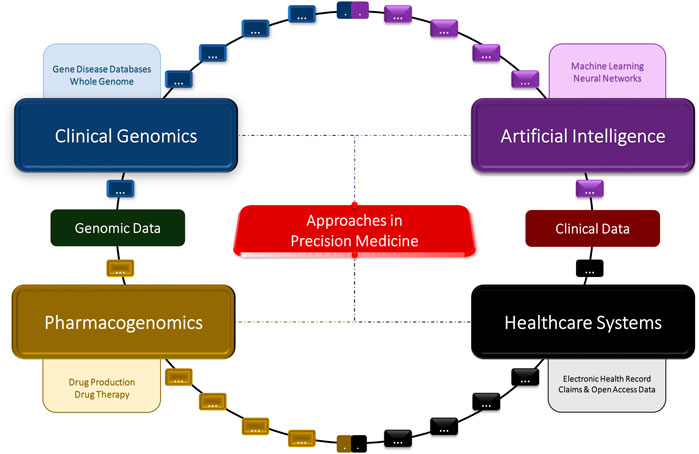
The rapid advancements in gene editing technologies and artificial intelligence (AI) are reshaping the landscape of modern medicine. These innovations are poised to revolutionize how we understand, diagnose, and treat a myriad of diseases, offering unprecedented potential to improve human health. This article delves into the synergistic potential of gene editing technologies, such as CRISPR-Cas9, and AI in medicine, exploring their current applications, future prospects, ethical considerations, and the challenges they face.
UNDERSTANDING GENE EDITING TECHNOLOGIES
CRISPR-CAS9: A REVOLUTIONARY TOOL
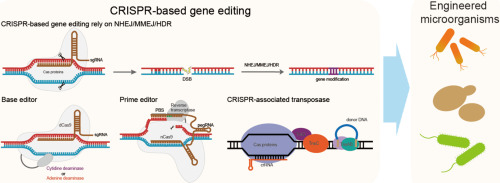
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPRassociated protein 9 (Cas9) is one of the most prominent gene-editing tools. Discovered in bacteria as an adaptive immune system, CRISPR-Cas9 allows scientists to edit DNA with remarkable precision. This technology works by guiding the Cas9 enzyme to a specific location in the genome, where it creates a double-strand break in the DNA. The cell’s natural repair mechanisms then take over, allowing for the addition, deletion, or modification of genetic material.
CRISPR-Cas9 has transformed genetic research due to its simplicity, efficiency, and versatility. Its applications range from basic research to potential therapeutic uses, making it a cornerstone of modern biotechnology.
OTHER GENE EDITING TECHNOLOGIES
While CRISPR-Cas9 garners much attention, other gene-editing technologies also play crucial roles. These include:
- ZINC FINGER NUCLEASES (ZFNS): Custom-designed proteins that bind to specific DNA sequences and create double-strand breaks, enabling targeted gene editing.
- TRANSCRIPTION ACTIVATOR-LIKE EFFECTOR NUCLEASES (TALENS): Similar to ZFNs, TALENs use engineered proteins to recognize and cut specific DNA sequences.
- BASE EDITORS AND PRIME EDITORS: These newer tools offer more precise editing capabilities by directly converting one DNA base into another or allowing for more controlled editing without creating double-strand breaks.
ARTIFICIAL INTELLIGENCE IN MEDICINE
MACHINE LEARNING AND DEEP LEARNING
AI encompasses a range of technologies, with machine learning (ML) and deep learning (DL) being the most relevant to medicine. ML algorithms learn from data to make predictions or decisions without explicit programming. DL, a subset of ML, involves neural networks with many layers (hence “deep”) that can learn complex patterns in data.
APPLICATIONS OF AI IN MEDICINE
AI’s applications in medicine are vast and varied, including:
- DIAGNOSTICS: AI algorithms can analyze medical images (such as X-rays, MRIs, and CT scans) to detect diseases like cancer with accuracy comparable to human experts. For example, AI systems can identify lung nodules or diabetic retinopathy, aiding early diagnosis and treatment.
- DRUG DISCOVERY: AI accelerates drug discovery by predicting how different molecules will interact with biological targets. This reduces the time and cost of bringing new drugs to market.
- PERSONALIZED MEDICINE: AI can analyze large datasets, including genetic information, to tailor treatments to individual patients. This approach promises more effective therapies with fewer side effects.
- PREDICTIVE ANALYTICS: By analyzing electronic health records (EHRs), AI can predict patient outcomes, such as the likelihood of readmission or disease progression, enabling proactive care.
- ROBOTICS AND SURGERY: AI-powered robots assist in surgeries, improving precision and reducing recovery times. These systems can perform minimally invasive procedures with higher accuracy than human surgeons.
THE SYNERGY OF GENE EDITING AND AI
The convergence of gene editing technologies and AI has the potential to create a new era in medicine. Here are several ways in which these technologies complement each other:
ACCELERATING GENETIC RESEARCH
AI can analyze vast amounts of genomic data to identify patterns and associations that might be missed by human researchers. This capability is crucial in understanding complex genetic diseases and identifying potential targets for gene editing. For instance, AI algorithms can sift through genomic datasets to pinpoint mutations linked to specific diseases, guiding CRISPR-Cas9 interventions.
ENHANCING PRECISION MEDICINE
Gene editing offers the promise of personalized treatments by directly modifying the genetic causes of diseases. AI can enhance this precision by predicting which genetic changes will produce the desired therapeutic outcomes. Machine learning models can simulate the effects of various gene edits, reducing the risk of unintended consequences.
DRUG DEVELOPMENT
Combining gene editing and AI can revolutionize drug development. AI can identify potential drug targets by analyzing genetic data, while gene editing can create accurate disease models for testing new therapies. This integrated approach can streamline the development of gene-based therapies and improve their safety and efficacy.
OVERCOMING BIOLOGICAL CHALLENGES
Gene editing faces several biological challenges, such as off-target effects and the delivery of editing tools to specific tissues. AI can help address these issues by optimizing guide RNA sequences to minimize off-target effects and designing delivery systems that ensure gene editors reach their intended targets.
CURRENT AND FUTURE APPLICATIONS
GENETIC DISEASES
Gene editing technologies hold immense potential for treating genetic diseases.
Conditions such as cystic fibrosis, sickle cell anemia, and muscular dystrophy are caused by specific genetic mutations. CRISPR-Cas9 and other gene-editing tools can correct these mutations, offering the possibility of permanent cures.
CANCER
Cancer treatment is another area where gene editing and AI can make a significant impact. Gene editing can be used to modify immune cells to better recognize and attack cancer cells, a technique known as CAR-T cell therapy. AI can identify new targets for these therapies and predict patient responses, making treatments more effective and personalized.
INFECTIOUS DISEASES
Gene editing has the potential to combat infectious diseases by modifying the genomes of pathogens or the host’s immune cells. For example, CRISPR-based antiviral therapies can target and destroy viral DNA or RNA within infected cells. AI can aid in the design of these therapies by predicting how viruses will evolve and identifying potential resistance mechanisms.
RARE DISEASES
Rare diseases often lack effective treatments due to limited understanding and funding. AI can accelerate research by identifying genetic causes and potential therapies, while gene editing can provide precise treatments tailored to individual patients. This approach offers hope to millions of people suffering from rare genetic disorders.
REGENERATIVE MEDICINE
Gene editing and AI are also advancing regenerative medicine. AI can optimize the differentiation of stem cells into specific cell types, while gene editing can correct genetic defects in patient-derived cells. This combination holds promise for developing personalized cell therapies and tissue engineering.
ETHICAL AND SOCIAL CONSIDERATIONS
ETHICAL CONCERNS
The power of gene editing technologies and AI raises several ethical concerns. These include:
- GERMLINE EDITING: Editing the genomes of embryos or germline cells can have heritable effects, raising concerns about unintended consequences and the potential for “designer babies.” The ethical implications of such interventions are still hotly debated.
- EQUITY AND ACCESS: Advanced medical technologies can exacerbate existing health disparities if not accessible to all. Ensuring equitable access to gene editing and AI-driven therapies is a major challenge.
- DATA PRIVACY: The use of AI in medicine involves the collection and analysis of vast amounts of personal health data. Ensuring the privacy and security of this data is crucial to maintaining public trust.
- REGULATORY OVERSIGHT: The rapid pace of innovation in gene editing and AI requires robust regulatory frameworks to ensure safety and efficacy. Balancing innovation with appropriate oversight is a complex task.
SOCIAL IMPLICATIONS
The integration of gene editing and AI in medicine will have profound social implications. These technologies can change how we perceive and manage health and disease, potentially leading to a shift in societal norms and expectations. Public engagement and education are essential to address misconceptions and foster informed discussions about the benefits and risks of these technologies.
CHALLENGES AND FUTURE DIRECTIONS
TECHNICAL CHALLENGES
Despite their potential, gene editing technologies and AI face several technical challenges:
- OFF-TARGET EFFECTS: Ensuring the specificity of gene editing tools to minimize unintended genetic changes is crucial. Advances in AI can help improve the accuracy of these tools, but further research is needed.
- DELIVERY SYSTEMS: Developing safe and efficient delivery systems for gene editing tools remains a significant challenge. AI can aid in designing nanoparticles or viral vectors that target specific tissues, but practical implementation requires extensive testing.
- SCALABILITY: Scaling up gene editing and AI-driven therapies for widespread clinical use involves significant logistical and manufacturing challenges. Ensuring consistent quality and safety across large-scale applications is essential.
REGULATORY AND ETHICAL FRAMEWORKS
Establishing robust regulatory and ethical frameworks is critical to the responsible development and deployment of gene editing and AI technologies. Policymakers, scientists, and ethicists must collaborate to create guidelines that balance innovation with safety, efficacy, and ethical considerations. International cooperation is also necessary to address cross-border issues and ensure global standards.
INTERDISCIPLINARY COLLABORATION
The convergence of gene editing and AI in medicine requires interdisciplinary collaboration. Biologists, geneticists, computer scientists, ethicists, and clinicians must work together to leverage the full potential of these technologies. Collaborative efforts can accelerate research, optimize therapeutic approaches, and address ethical and social challenges.
EDUCATION AND PUBLIC ENGAGEMENT
Educating the public about the potential and limitations of gene editing and AI is crucial for informed decision-making and societal acceptance. Transparent communication about the benefits, risks, and ethical considerations of these technologies can foster public trust and support. Engaging with diverse stakeholders, including patients, advocacy groups, and policymakers, can ensure that the development of these technologies aligns with societal values and needs.
TECHNICAL ADVANCEMENTS AND INNOVATIONS
IMPROVING OFF-TARGET EFFECTS
One of the critical technical challenges of gene editing, particularly with CRISPR-Cas9, is minimizing off-target effects. These unintended edits can lead to unpredictable consequences, potentially causing more harm than good. Researchers are continuously working on enhancing the specificity of CRISPR-Cas9 by developing improved versions of the enzyme and more precise guide RNAs. AI plays a significant role in this process by predicting off-target sites and optimizing guide RNA sequences to minimize these risks. Machine learning algorithms can analyze vast amounts of genomic data to predict the most likely off-target sites, thereby guiding the design of more accurate CRISPR tools.
ENHANCING DELIVERY SYSTEMS
Efficient delivery of gene editing tools to the target cells and tissues remains a major hurdle. Current methods include viral vectors, lipid nanoparticles, and physical methods like electroporation. Each method has its limitations in terms of efficiency, safety, and specificity. AI can aid in designing better delivery systems by modeling and simulating the interactions between delivery vehicles and biological systems. For instance, AI can predict the best lipid compositions for nanoparticles that can effectively deliver CRISPR components to specific tissues without causing adverse effects.
HIGH-THROUGHPUT SCREENING AND AI
High-throughput screening (HTS) is a technique used to quickly conduct millions of chemical, genetic, or pharmacological tests. Combining HTS with AI allows for the rapid identification of potential gene targets and therapeutic compounds. AI algorithms can analyze the vast datasets generated by HTS to identify patterns and correlations that might indicate a successful gene edit or therapeutic intervention. This approach can significantly speed up the discovery of new drugs and the development of gene therapies.
ETHICAL AND SOCIAL DIMENSIONS
GERMLINE EDITING AND ITS IMPLICATIONS
One of the most controversial aspects of gene editing is germline editing, which involves making changes to the DNA of embryos or reproductive cells. These changes are heritable and can be passed on to future generations. While germline editing holds the promise of eradicating genetic diseases, it also raises significant ethical concerns. The potential for unintended consequences, the possibility of creating “designer babies,” and the long-term impacts on the human gene pool are all issues that need careful consideration. Robust ethical guidelines and regulatory frameworks are essential to ensure that germline editing is conducted responsibly and ethically.
ENSURING EQUITY AND ACCESS
The development of advanced medical technologies often leads to concerns about access and equity. There is a risk that gene editing and AI-driven therapies could widen existing health disparities if they are only available to those who can afford them. Ensuring equitable access to these technologies is crucial for their widespread adoption and acceptance. This requires concerted efforts from policymakers, healthcare providers, and the biotech industry to develop affordable treatments and ensure that they are accessible to all, regardless of socioeconomic status.
DATA PRIVACY AND SECURITY
AI in medicine relies heavily on the analysis of large datasets, including sensitive personal health information. Ensuring the privacy and security of this data is paramount to maintaining public trust. Robust data protection measures, such as encryption and anonymization, are essential to safeguard patient information. Additionally, transparent policies regarding data use and sharing can help build trust and ensure that patients feel confident in the security of their health data.
FUTURE DIRECTIONS AND EMERGING TRENDS
INTEGRATION OF MULTI-OMICS DATA
The integration of multi-omics data (genomics, proteomics, transcriptomics, etc.) with AI represents a promising frontier in precision medicine. By analyzing data from multiple biological layers, AI can provide a more comprehensive understanding of disease mechanisms and identify novel therapeutic targets. For example, integrating genomic data with proteomic data can help elucidate how genetic mutations affect protein expression and function, leading to more effective treatments.
AI-DRIVEN DRUG REPURPOSING
Drug repurposing involves finding new uses for existing drugs. AI can analyze large datasets of drug interactions, side effects, and patient outcomes to identify existing drugs that could be effective against other diseases. This approach can significantly reduce the time and cost of drug development, as repurposed drugs have already undergone extensive safety testing. AI-driven drug repurposing has the potential to accelerate the availability of new treatments for diseases that currently lack effective therapies.
PERSONALIZED GENE THERAPY
Personalized gene therapy aims to tailor treatments to the individual genetic makeup of patients. AI can help identify the specific genetic mutations responsible for a patient’s disease and predict how different gene editing strategies might correct these mutations. This personalized approach can lead to more effective and safer treatments, as therapies are designed to target the precise genetic causes of disease in each patient.
CASE STUDIES AND REAL-WORLD APPLICATIONS
SICKLE CELL DISEASE AND BETA-THALASSEMIA
Sickle cell disease and beta-thalassemia are genetic blood disorders caused by mutations in the hemoglobin gene. Researchers have used CRISPR-Cas9 to edit the genomes of patients’ hematopoietic stem cells, correcting the mutations responsible for these diseases. Clinical trials have shown promising results, with patients experiencing significant improvements in symptoms and quality of life. AI has played a crucial role in optimizing the gene editing process, predicting off-target effects, and monitoring patient outcomes.
CANCER IMMUNOTHERAPY
CAR-T cell therapy, a form of cancer immunotherapy, involves modifying a patient’s T cells to express a chimeric antigen receptor (CAR) that targets cancer cells. AI has been instrumental in designing CARs with high specificity and affinity for cancer antigens. Additionally, AI-driven predictive models can identify patients who are most likely to benefit from CAR-T cell therapy and monitor their response to treatment, leading to more personalized and effective cancer therapies.
RARE GENETIC DISORDERS
Rare genetic disorders often lack effective treatments due to limited understanding and funding. However, AI and gene editing technologies are changing this landscape. For example, researchers are using AI to analyze genomic data from patients with rare diseases, identifying potential genetic causes and therapeutic targets. Gene editing tools, such as CRISPR-Cas9, can then be used to correct these genetic defects, offering hope to patients with conditions that were previously considered untreatable.
EMERGING APPLICATIONS AND FUTURE PROSPECTS
The potential applications of gene editing technologies and artificial intelligence in medicine are vast and continue to grow as both fields evolve. As research progresses and new technologies emerge, several exciting areas of development and exploration offer hope for transformative changes in healthcare.
GENE EDITING IN VIRAL INFECTIONS
Gene editing technologies like CRISPR-Cas9 have shown potential in combating viral infections. One of the most significant challenges in treating viral diseases, such as HIV and Hepatitis B, is the ability of viruses to integrate their genetic material into the host genome. This makes it difficult to eliminate the virus completely, as conventional antiviral therapies cannot target the integrated viral DNA.
Researchers have demonstrated that CRISPR can be used to target and excise viral DNA from infected cells, effectively “curing” infections in laboratory settings. AI can assist in predicting which segments of the viral genome are most vulnerable to editing and how to optimize CRISPR for the best chance of success. This combination of gene editing and AI may lead to novel treatments for persistent viral infections that could revolutionize the way we approach viral diseases.
GENE EDITING AND EPIGENETIC REGULATION
Epigenetics refers to changes in gene expression that do not involve alterations in the underlying DNA sequence but are still passed down through generations. These modifications can significantly impact health, leading to conditions such as cancer, neurological diseases, and metabolic disorders. Gene editing, when combined with an understanding of epigenetic mechanisms, could offer powerful therapeutic options.
AI can analyze the complex patterns of gene expression and epigenetic modifications to identify targets for gene editing that may reverse harmful epigenetic changes. By using
CRISPR and other gene-editing tools to modify epigenetic regulators, scientists could “reset” abnormal gene expression profiles associated with disease, offering potential cures for conditions that are traditionally difficult to treat.
AI IN MANAGING COMPLEX DISEASES AND MULTIMORBIDITY
As the global population ages, the prevalence of multimorbidity (the coexistence of multiple chronic conditions) is increasing. Managing these complex conditions is a significant challenge for healthcare providers, as patients may require individualized treatment regimens that consider the interaction of various diseases and medications.
AI, particularly in predictive modeling and data analysis, can help identify patterns in patient data that indicate which treatments may work best for those with multiple health conditions. By integrating genetic data with clinical data (such as electronic health records, imaging, and lifestyle information), AI can help develop personalized treatment strategies that address the needs of multimorbid patients, improving outcomes and reducing healthcare costs.
AI can also assist in real-time monitoring of patient health and automatically adjust treatment plans based on evolving health conditions, offering more proactive and dynamic management of complex diseases.
AI-DRIVEN CLINICAL TRIALS AND GENE EDITING THERAPIES
Clinical trials are a crucial step in developing new gene therapies and treatments, but they are often expensive, time-consuming, and difficult to design effectively. AI can be used to streamline the clinical trial process by analyzing historical clinical data and predicting which patients are most likely to benefit from a specific treatment. Machine learning algorithms can also help design more efficient trials by identifying the optimal trial designs and reducing the number of participants needed.
In gene editing therapies, where individual patients may require customized treatment plans, AI-driven simulations can predict how specific genetic edits will affect patient outcomes. By optimizing the design of gene-editing trials, AI can accelerate the approval process for new therapies, bringing innovative treatments to market faster and more safely.
ARTIFICIAL INTELLIGENCE IN HEALTHCARE ROBOTICS AND SURGERY
AI-powered robots are transforming surgery by enhancing precision, reducing errors, and enabling minimally invasive procedures. AI-driven robotic systems, such as those used in robotic-assisted surgery, provide surgeons with greater control over instruments during operations, particularly in complex or delicate procedures.
Gene editing could complement AI-powered surgical robots by providing real-time, precise adjustments to a patient’s genetic makeup during surgery. For example, if a surgical procedure uncovers genetic defects or disease markers, the robot could employ geneediting tools to correct these abnormalities immediately. This integration of AI, robotics, and gene editing could pave the way for personalized, on-the-spot medical interventions that target genetic diseases during surgery itself.
BIOENGINEERING AND ORGAN REGENERATION
infrastructure, and insufficient medical expertise. However, gene editing and AI could help level the playing field.
AI-driven telemedicine platforms, for example, could extend diagnostic capabilities to remote and underserved regions by enabling healthcare providers to leverage AI for disease detection, even in areas with limited access to specialized medical expertise. Similarly, gene editing technologies could be used to develop more cost-effective treatments for genetic diseases, such as sickle cell anemia, which disproportionately affect populations in Africa and South Asia.
The key challenge is ensuring that these technologies are made affordable and accessible to everyone, especially in resource-poor settings. Public-private partnerships, international cooperation, and funding models will be essential in ensuring that the benefits of gene editing and AI are shared equitably across the globe.
GLOBAL PUBLIC HEALTH
Gene editing and AI also have the potential to play a significant role in global public health initiatives, such as the fight against infectious diseases and the prevention of genetic disorders. AI can assist in monitoring disease outbreaks by analyzing epidemiological data and predicting where outbreaks are most likely to occur. By combining AI’s predictive capabilities with gene editing’s ability to eradicate genetic diseases or correct mutations, the global health community can tackle both infectious diseases (such as malaria and tuberculosis) and non-communicable diseases (such as genetic disorders).
In the future, AI may be used to predict and mitigate the spread of pandemics by analyzing genetic mutations in pathogens and identifying potential therapeutic targets. AI can also assist in the distribution of vaccines and treatments to ensure that they reach the populations most at risk.
ETHICAL OVERSIGHT AND GOVERNANCE
As the power of gene editing and AI in medicine grows, so too does the need for ethical oversight and governance. While these technologies have enormous potential to improve human health, they also present risks and challenges that must be carefully managed. International bodies, governments, and regulatory agencies must work together to create global standards for the responsible use of these technologies.
Key issues, such as the use of gene editing for enhancement (rather than therapeutic purposes), the regulation of AI in healthcare, and the management of genetic data privacy, must be addressed through transparent and robust ethical guidelines. Public involvement in discussions around these issues is essential to ensure that the development and deployment of gene editing and AI in medicine align with societal values.
CONCLUSION
The potential of gene editing technologies and artificial intelligence to transform medicine is enormous, with the ability to enhance our understanding of diseases, revolutionize treatment strategies, and improve patient outcomes. As these technologies continue to advance, the possibilities for personalized and precise healthcare become increasingly accessible. By combining the power of CRISPR and other gene-editing tools with AI-driven data analysis, we are entering an era where medical care is not only more targeted but also more proactive and effective.
However, the journey toward fully realizing the potential of gene editing and AI in medicine will require overcoming significant challenges—technical, ethical, and social. While there is enormous promise in these innovations, careful consideration of their broader implications, particularly in terms of equity, access, and regulation, is necessary to ensure that they benefit all of humanity.
As we move forward, collaboration across disciplines, nations, and industries will be critical to shaping a future in which gene editing and AI contribute to a healthier, more equitable world. With careful planning and responsible stewardship, these groundbreaking technologies will undoubtedly unlock new frontiers in medicine and improve the lives of millions of people globally.